Our current research interests in nanoparticle (NP) chemistry cover two general aspects: (1) chemical synthesis and self-assembly of NPs; (2) elaboration of functional NPs for applications in catalysis, green chemistry, functional materials, and renewable energy.
Nanoparticle Synthesis and Self-Assembly
We utilize the so-called “bottom-up” approach to synthesize monodisperse NPs. By controlling chemical reaction conditions in solutions, we can have atoms “stacked” in certain dimensions and directions. As a result, we are able to tune NP sizes, compositions, structures and shapes. These NPs stabilized by surfactants are readily dispersed in solvents, facilitating their further surface functionalization or self-assembly into 2D or 3D superlattice structures with controlled NP packing densities, interparticle interactions, and synergistic effects. We are investigating these NPs and their assemblies for applications in magnetic data/energy storage and catalysis for green chemistry and sustainable energy. Figure 1 shows a few transmission electron microscopy images of some NPs we prepared in our lab, highlighting the NP quality control we have achieved in the chemical syntheses.
 Figure 1. TEM images of some representative NPs we prepared, including polyhedral, hollow, nanotube, dumbbell-like NPs and Au nanowires.
Figure 1. TEM images of some representative NPs we prepared, including polyhedral, hollow, nanotube, dumbbell-like NPs and Au nanowires.
Stable Nanoparticles for Electrocatalysis
NP catalysis can be controlled by NP dimensions, compositions and structures. One of our current focal studies is to control chemical stability of alloy NPs so that alloy effects on catalysis can be better investigated for electrochemical reduction and oxidation reactions that are key to water splitting, fuel cell and metal-air battery applications. We have demonstrated that Fe in the FePt structure can be efficiently stabilized in the tetragonal intermetallic structure in which Fe atoms are sandwiched by Pt atoms via strong d-orbital interaction along the crystallographic c direction (Figure 2A), and efficiently stabilized against oxidation and acid etching. The stabilization of Fe in the FePt structure further promotes Pt catalysis (Figure 2B) for oxygen reduction reaction (ORR) (Figure 2C&D) and hydrogen evolution reaction (HER). This concept has been extended to various intermetallic NPs for ORR catalysis in the fuel cell operation conditions. When the intermetallic NPs are alloyed with Au on their surfaces, they become active for electrochemical oxidation of formic acid and alcohols. This controlled surface modification of intermetallic alloy NPs provides a rational approach to enhancing alloy NP electrocatalysis for either reduction or oxidation reactions.
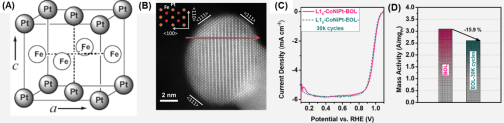 Figure 2. (A) Schematics of tetragonal intermetallic FePt structure. (B) A HRTEM image of an intermetallic core/shell FePt NP coated with 2 atomic layer of Pt. (C, D) ORR catalysis performance of a CoNiPt intermetallic NP catalyst, showing much enhanced activity and stability (BOL = begin of life; EOL = end of life).
Figure 2. (A) Schematics of tetragonal intermetallic FePt structure. (B) A HRTEM image of an intermetallic core/shell FePt NP coated with 2 atomic layer of Pt. (C, D) ORR catalysis performance of a CoNiPt intermetallic NP catalyst, showing much enhanced activity and stability (BOL = begin of life; EOL = end of life).
Nanoparticle Catalysis for CO2 Reduction
Selective reduction of CO2 is an important step to achieve chemical/fuel sustainability. With controlled NP synthesis and assembly, we are in a unique position to study NP electrocatalysis for CO2 reduction. We studied Au NP catalysis and found Au edge sites on the Au NP surface to be especially selective for CO2 reduction to CO (see the Figure below). We also found Cu nanowires (NWs) to be selective for CO reduction to hydrocarbons. Using molecule-assisted assembly, we anchored Au NPs onto Cu NWs via bipyridine (see the right Figure) and prepared a composite catalyst to catalyze the CO2 reduction to CO and then CO reduction to hydrocarbon products with a total Faradaic efficiency (FE) reaching 90.6%. We recently extended our studies to ferromagnetic 1.3 nm thick hcp-Co nanosheets (NSs), and found that this NS catalyst was very active and selective for CO2 reduction to CH3CHO, CH3CH2OH and CH3OH with a total FE of 82%. We will continue to investigate the synergistic effects among different nanocatalyst components to improve CO2 reduction activity and selectivity. Our goal is to maximize NP catalysis for the reduction of CO2 that is directly captured from air.
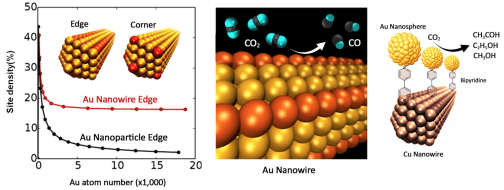 Figure 3. (left) A plot showing the edge and cover site densities along a Au nanowire, (middle) Au nanowire-catalyzed CO2 conversion to CO, (right) Au NPs assembled on Cu nanowires via 1,4-bipyrridine for improved CO2 reduction selectivity via synergistic catalysis between Au and Cu.
Figure 3. (left) A plot showing the edge and cover site densities along a Au nanowire, (middle) Au nanowire-catalyzed CO2 conversion to CO, (right) Au NPs assembled on Cu nanowires via 1,4-bipyrridine for improved CO2 reduction selectivity via synergistic catalysis between Au and Cu.
Nanoparticle-Catalyzed Tandem Reactions
We have also extended the NP catalysis to green chemistry reactions. Our current interests are in exploring NP ability to catalyze multiple chemical reactions in one-pot and green chemistry conditions. Below is an example we have studied in using Cu NPs as a robust catalyst for chemo-selective reduction of 3-nitrostyrene to 3-vinylaniline. We have extended the one-pot reaction approach to prepare other functional molecules and polymers, including polybenzoxazole (PBO), a rigid-rod organic polymer that is thermo-chemically stable and mechanically robust for potential ballistic fiber, anti-flame coating and heat-resistant membrane applications.
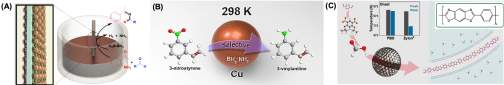 Figure 4 (A) Illustration of self-assembled monolayer NP array as a probe to catalyze multistep chemical reactions in one-pot. (B) Schematic of Cu NP-catalyzed chemo-selective reduction of 3-nitrostyrene to 3-vinylaniline. (C) Schematic of AuPd NP-catalyzed one-pot reactions, leading to the formation of highly pure PBO with much enhanced thermo-mechanical stability than the commercial PBO (Zylon).
Figure 4 (A) Illustration of self-assembled monolayer NP array as a probe to catalyze multistep chemical reactions in one-pot. (B) Schematic of Cu NP-catalyzed chemo-selective reduction of 3-nitrostyrene to 3-vinylaniline. (C) Schematic of AuPd NP-catalyzed one-pot reactions, leading to the formation of highly pure PBO with much enhanced thermo-mechanical stability than the commercial PBO (Zylon).

